Roberts et al. carried out an important experiment to prove the existence of benzine. Chlorobenzene labeled with 14C at the position to which the chlorine was attached was treated with sodium amide in liquid ammonia and the aniline formed was analyzed for the 14C position. The results are indicated in the figure. Show how these data are consistent with the benzyne intermediate.
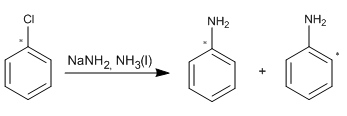
SOLUTION:
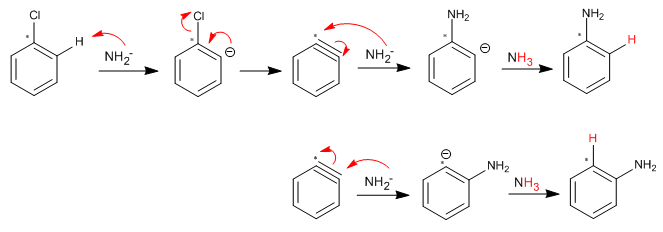
Halogenated benzenes lacking ortho/para deactivating groups react with nucleophiles via the nucleophilic aromatic substitution (elimination-addition) mechanism. This reaction occurs through an intermediate called benzyne.