For each of the following pairs of molecules (or reactions), indicate which will take place more rapidly. Reason the answer.
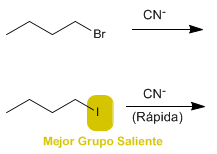
a) 1-Bromobutane or 1-iodobutane with sodium cyanide in DMSO.
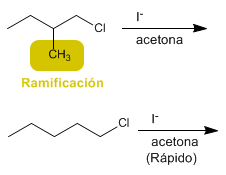
b) 1-Chloro-2-methylbutane or 1-chloropentane with sodium iodide in acetane
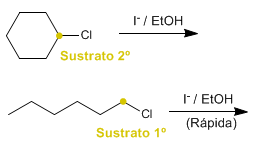
c) 1-Chlorohexane or chlorocyclohexane with sodium iodide in ethanol/water.
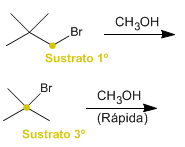
d) Solvolysis of 1-bromo-2,2-dimethylpropane or 2-bromo-2-methylpropane in methanol
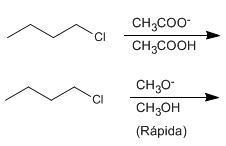
e) 1-Chlorobutane with sodium acetate in acetic acid or with sodium methoxide in methanol.
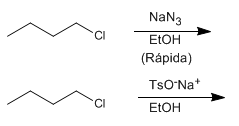
f) 1-Chlorobutane with sodium azide in aqueous ethanol or sodium p-toluenesulfonate in aqueous ethanol.
SOLUTION:
a) 1-Iodobutane reacts faster, as iodine is the best leaving group than bromine.
b) Branches at carbon b slow down SN2.
c) The primary substrate (1-chlorohexane) reacts faster than the secondary
d) Tertiary substrates react faster than primary ones in SN1
e) Methoxide is a better nucleophile than acetate and reacts faster.
f) The azide ion, much more basic and nucleophilic than sodium tosylate, reacts faster.