In nucleophilic substitution reactions (SN2), one group, called the leaving group, is exchanged for another, called the nucleophile. 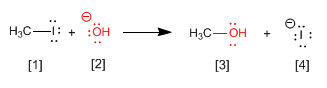
[1] Substrate - Species containing the leaving group
[2] Nucleophile - Lewis base capable of attacking atoms with positive polarity (or charge)
[3] Reaction product
[4] Leaving group - Species that leaves the substrate, being replaced by the nucleophile
Nucleophilic substitution reactions allow many organic compounds to be obtained from alkyne halides. Let's see some examples:
a) The reaction of bromoethane with sodium hydroxide produces ethanol. ![]()
b) The reaction of isopropyl chloride with sodium cyanide produces a nitrile 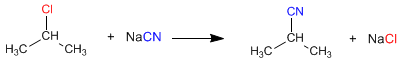
c) The reaction of iodomethane with sodium methoxide produces an ether (dimethyl ether) ![]()
d) Amines can be obtained by reacting ammonia with haloalkanes ![]()
In these examples the leaving group is represented in red and the nucleophile in blue.