There is a significant speed difference between the substrates; methyl bromide, ethyl bromide, isopropyl bromide and tert-butyl bromide when reacted with a nucleophile under the same conditions.
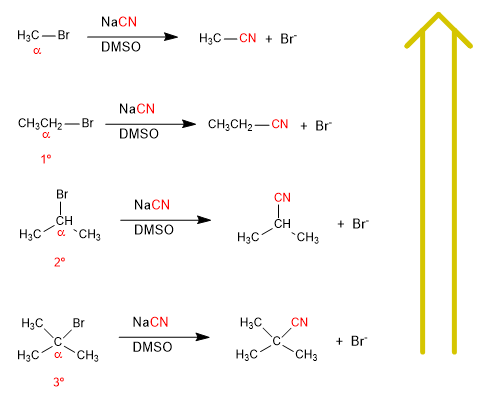
As you move up the list of reactions, the speed increases.
The carbon attached to the leaving group is called a , and substrates are classified as primary, secondary, or tertiary depending on whether the a-carbon has one, two, or three carbon chains.
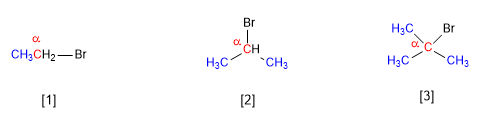
[1] Primary substrate (Reacts fast in SN2)
[2] Secondary substrate (Reacts slowly in SN2)
[3] Tertiary substrate (Does not react in SN2)
The SN2 mechanism involves attack by the nucleophile on the side opposite the leaving group, dorsal attack. The carbon chains impede this face of the substrate, producing a decrease in speed as the number of chains increases.
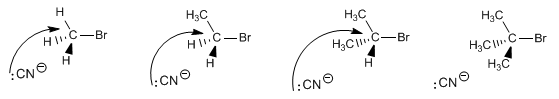
As the number of carbon chains around carbon a increases, the attack of the nucleophile becomes more complicated, becoming unfeasible in the case of the tertiary substrate.
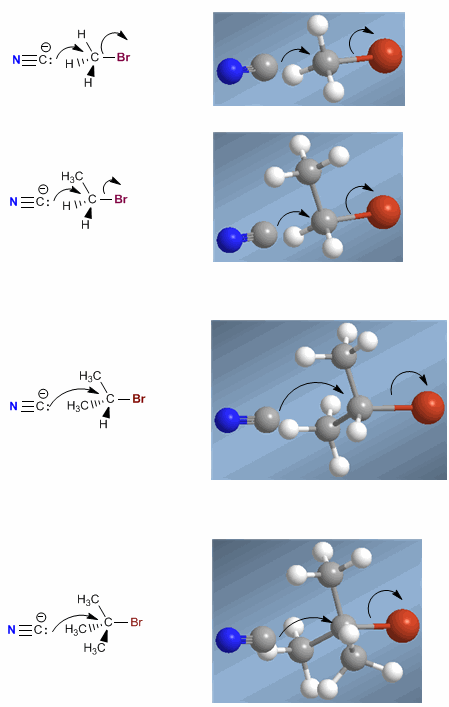
In the tertiary substrate the three methyls surrounding carbon a totally prevent the approach of the nucleophile and SN2 does not take place.