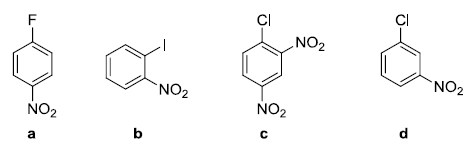
Arrange the following compounds in increasing order of nucleophilic aromatic substitution reaction rate:
SOLUTION:
d
The rate of aromatic nucleophilic substitution by the addition-elimination mechanism depends on the number of deactivating groups located in ortho/para positions and the type of halogen, following the order F> Cl> Br> I.
Molecule (c) is the most reactive because it has two nitro groups in ortho/para. Molecule (d) is the least reactive as it has the nitro group in meta.