Since the slow step of unimolecular nucleophilic substitution (SN1) is dissociation of the substrate, and the nucleophile acts in the second step, the rate of the reaction does not depend on the nucleophile. The following three reactions proceed at the same speed since they start from the same substrate.
Obviously, it is necessary that other factors, such as solvent, be the same in the three reactions
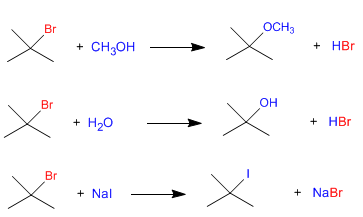
All three reactions proceed at the same speed, the slow pace is the same for all.
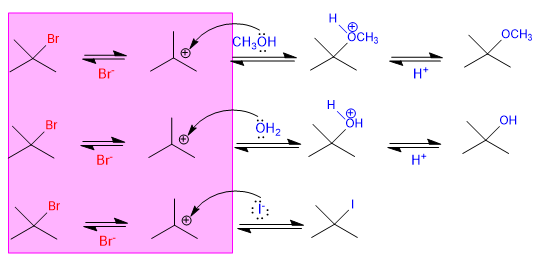
Slow stage common to the three mechanisms
In the case of competition between two nucleophiles, the better one captures most of the carbocations, giving the majority product.
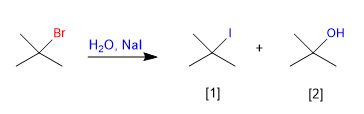
[1] Majority product (Iodide is better nucleophile than water)
[2] Minority product