The pyridine ring is capable of attacking electrophiles, analogously to benzene, giving the aromatic electrophilic substitution reaction. Due to the electronegativity of nitrogen, pyridine is much less reactive than benzene in SE, requiring more drastic reaction conditions.
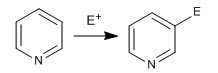
The favored positions are 3 and 5, due to the greater stability of the reaction intermediate.
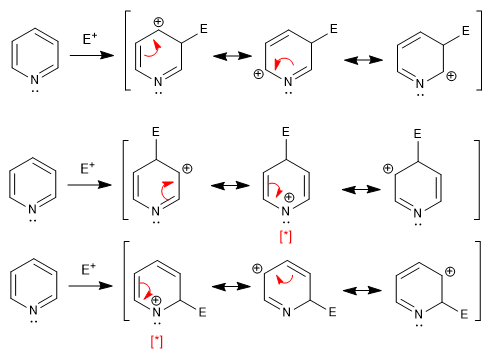
The resonance hybrid that results from placing the electrophile in position 2,4 is less favorable than that of position 3, due to the presence of a canonical structure with positive nitrogen and only six electrons.
Now we are going to see which reagents and conditions can be used in the electrophilic substitution.
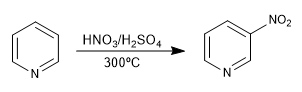
1. Nitration:
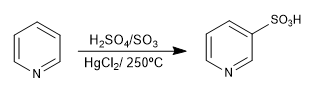
2. Sulfonation:
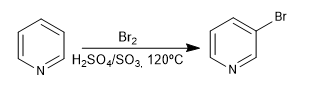
3. Bromation:
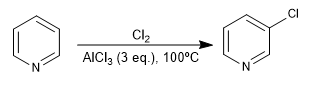
4. Chlorination:
Friedel-Crafts alkylations and acylations are not feasible as alkyl and acyl halides react on nitrogen.