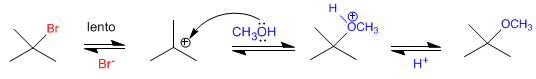The SN1 mechanism, analogous to the SN2 mechanism, requires good leaving groups.
TsO- > I- > Br- > H2O > Cl-
Water does not react with 2-Fluoropropane since fluorine is a bad leaving group, but it does with 2-Bromopropane.
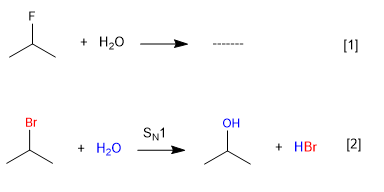
[1] Reaction does not take place (Fluorine bad leaving group)
The leaving group influences the rate of SN1, since the slow step in the mechanism is dissociation from the substrate. The better the leaving group, the faster the reaction.
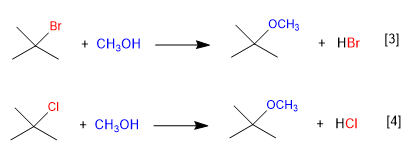
[3] Quick reaction
[4] Slow reaction (chlorine worse leaving group than fluorine)
tert-Butyl bromide ionizes faster than tert-Butyl chloride, because bromine is a better leaving group than chlorine. As the dissociation of the substrate is the slow step of the mechanism, the first reaction is faster than the second.