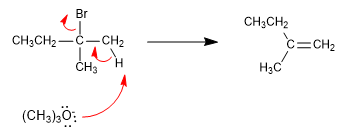
Alkenes can be prepared from haloalkanes and alkyl sulfonates by bimolecular elimination (E2). In the following example 2-bromo-2-methylbutane reacts with sodium methoxide to form a mixture of 2-methyl-2-butene and 2-methyl-1-butene.
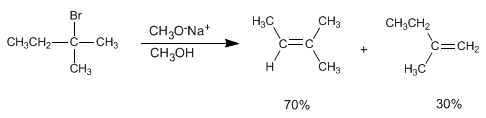
In this elimination, the most stable product (more substituted alkene) is obtained, and it is said that it follows Saytzev's rule .
The methoxide, small base, subtracts the innermost hydrogen from the haloalkane to produce the more stable product (thermodynamic alkene).
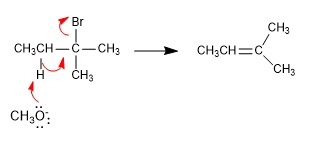
If we use tert-butoxide or LDA as the base, the alkene formed mainly is 2-methyl-1-butene. The hindered bases have difficulties in accessing the innermost hydrogen, subtracting the most accessible hydrogen faster, so they generate the less stable product for the most part. In this case the reaction is controlled kinetically and it follows Hofmann's rule .
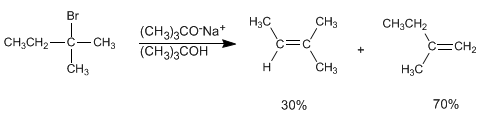
The hydrogens located on methyls are more accessible to tert-butoxide than the innermost hydrogens. The alkene formed mostly is the least substituted (kinetic alkene), and the reaction is said to follow Hofmann's rule.