A) Heterocycles with 6 atoms and 6 p electrons.
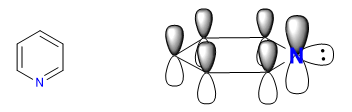
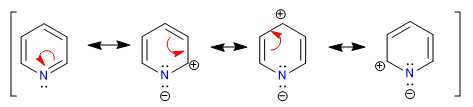
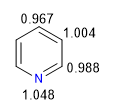
The more electronegative nitrogen draws the electron cloud towards it, leaving the electron-deficient carbons behind. Therefore, this system is considered pi deficient with respect to benzene. In the following image I represent the average charge densities that each carbon supports, in a benzene all carbons would support a density of 1.
The electronegativity of nitrogen causes the retention of the electronic cloud on it, decreasing the aromaticity of the system, as well as its ability to attack electrophiles. However, reactions that proceed with nucleophile attack on the ring are favored.
Eight types of aza compounds derived from benzene are known:
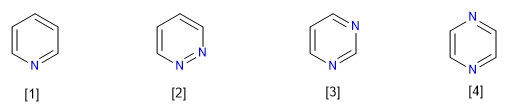
[1] Pyridine
[2] Pyridazine
[3] Pyrimidine
[4] Pyrazine
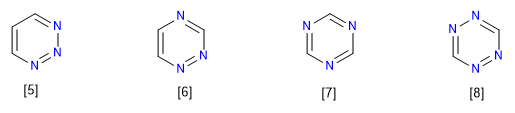
[5] 1,2,3-Triazine
[6] 1,2,4-Triazine
[7] 1,3,5-Triazine
[8] 1,2,3,5-Tetrazine
Other heterocycles belonging to this family with a heteroatom other than nitrogen are:
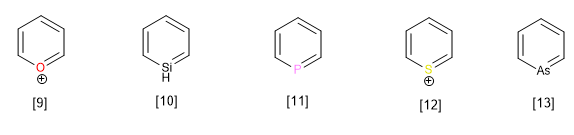
[9] Pyrilium cation
[10] Silabenzene
[11] Phosphabenzene
[12] Thiopyrilium cation
[13] Arsabenzene
B) Heterocycles of 5 atoms and 6 pi electrons.
They are compounds with electronic excess or p -surplus. Carbons have a higher charge density than benzene, this makes them very reactive towards electrophiles but drastically decreases their reactivity towards nucleophiles. A typical member of this family is pyrrole.
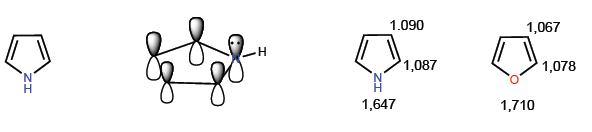
The most representative members of this family of heterocycles are:
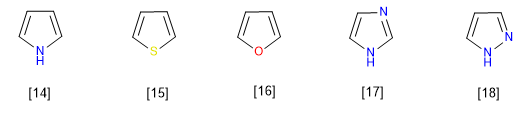
[14] Pyrrole
[15] Thiophene
[16] Furan
[17] Imidazole
[18] Pyrazole
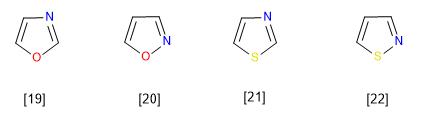
[19] Oxazole
[20] Isoxazole
[21] Thiazole
[22] Isothiazole
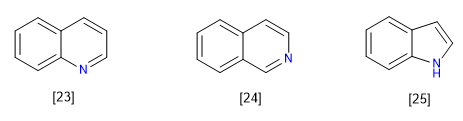
C) Condensed or fused systems