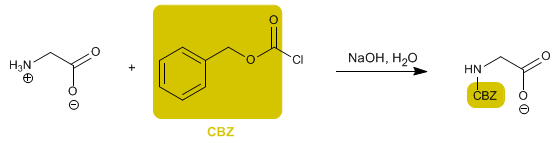
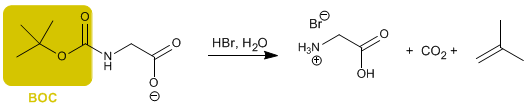The amino group is protected to prevent its reaction with the acid group of the second amino acid. Once the peptide bond is formed, deprotection is performed, again leaving the amino group free.
Benzyl chloroformate (CBZ-Cl) is used as a protective group for the amino, transforming it into an amide.
The deprotection of the amino group is carried out by palladium catalyzed hydrogenation.
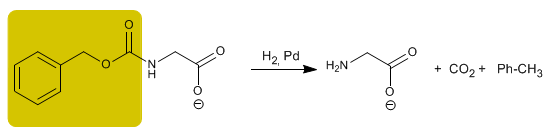
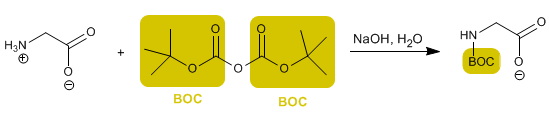
Another widely used reagent to protect the amino group is (BOC) 2 O
The deprotection of the group is carried out, in this case, with aqueous hydrobromic acid.