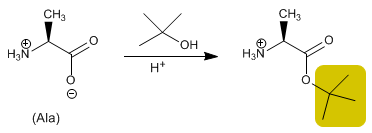
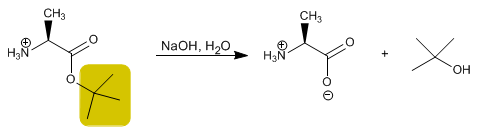
The acid group is protected by transforming it into an ester by reaction with an alcohol. Methyl, ethyl or tert -butyl esters are prepared by esterification and hydrolyzed (deprotected) in a basic medium.
Protection of alanine with tert-butanol in an acid medium.
Deprotection can be performed with acid or base hydrolysis of the ester.
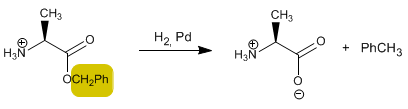
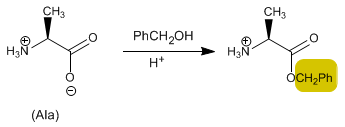
Another very interesting protecting group is benzyl alcohol. When reacting with the acid it forms an ester that can be cleaved by hydrogenation with H 2 /Pd. If the amino group is protected with CBZ-Cl and the acid with benzyl alcohol, both can be simultaneously deprotected by hydrogenation.
Deprotection by hydrogenation of the CO bond