Organic synthesis, the heart of organic chemistry , is an essentially heuristic activity, that is, it is a process where the highly predictive creative activities of logical thought and empirical procedures, rich in artistic elaboration, merge, making the organic chemist a true innovative.
Like any method, the " disconnection or synthon method " has its own structure, symbology and language, which must initially be assimilated and understood by those who are willing to use this synthetic tool.
The method of synthesis of the disconnections or synthon , includes Two phases;
![]() Retrosynthetic analysis phase . It shows all the transformations that will be carried out in the process of simplifying the structure of
Retrosynthetic analysis phase . It shows all the transformations that will be carried out in the process of simplifying the structure of
![]() Synthesis phase . Where what is "thought", based on criteria of mechanistic rationality and reactivity of organic compounds, materializes in a synthesis route, which will be written, as it is expected to occur in the chemical laboratory. It is where experience emerges and manifests the " art of doing or invent ” of the chemist, i.e.
Synthesis phase . Where what is "thought", based on criteria of mechanistic rationality and reactivity of organic compounds, materializes in a synthesis route, which will be written, as it is expected to occur in the chemical laboratory. It is where experience emerges and manifests the " art of doing or invent ” of the chemist, i.e.
The terms, definitions or synthesis operations, recurrently used in this method, are the following:
Target Molecule (MOb) .
This is the name given to any molecule that is to be synthesized or prepared from simple and affordable materials, which in a problem may be previously defined or adjusted to the options that the chemist generates in his synthesis plan or design.
transformation . ( ![]() ).
).
The special unidirectional retrosynthesis arrow should be understood as a symbolic representation of the expression “ is prepared from ” and also represents some kind of transformation in the structure of
The types of transformation referred to are actually retrosynthetic operations such as: Disconnections, Reconnections, Rearrangements, Interconversion of Functional Groups (IGF), Addition of Functional Groups (AGF), Deletion of Functional Groups (SGF), etc.
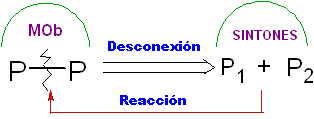
Disconnection.
It is a retrosynthetic operation that represents the imaginary breaking of the chemical bonds that would have been formed in the target molecule, from the synthons or more precisely from their synthetic equivalents (precursor molecules), postulated.
It can be understood as the inverse of a chemical reaction, it is represented by an arrow (very different from that of a chemical reaction or equilibrium conditions) and a crossed wavy line over the bond that will be "disconnected".
It is even possible to place the proposed disconnection on the arrow: CC, CS. CX, CO, CN. etc.. Expressions that link us to the type of reaction that will be used, in the formation of
In other cases, the disconnection model that is being used can be written, for example, it is common to find: 1, 3-diO, 1,4 –diCO, 1,5-diCO or α, β -insatCO . Etc.
Therefore, in a dioxygenated molecule, the following disconnections can be expected to occur:
![]() heterolytic disconnections,
heterolytic disconnections,
![]() Homolytic or radical disconnections
Homolytic or radical disconnections
![]() Electrocyclic Disconnections
Electrocyclic Disconnections
![]() Reorder Disconnects
Reorder Disconnects
It is the synthetic operation of reconnecting two points of
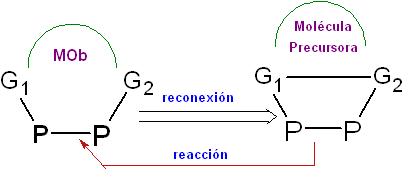
A typical example of reconnection is found in the Molecules that were obtained by the opening of a chemical bond, as is the case, for example, of the ozonolysis of alkenes, which provide products that for their synthesis must begin with the reconnection operation.
Rearrangement . The transform operation, which allows you to relocate a substructure of
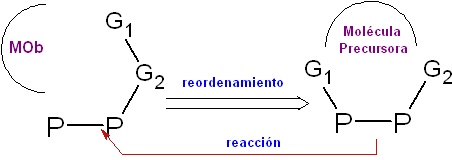
The pinacoline rearrangement, the Fries rearrangement, the Beckmann rearrangement, the Curtius rearrangement, and the Claisen rearrangement.
These are some typical examples of transposition, whose products will require a rearrangement of their structural components in the transformation operation, to reach their precursor molecule.
Retroelectrocyclic .
It is possible to postulate a simultaneous disconnection by two links, which are assumed to have been formed by a
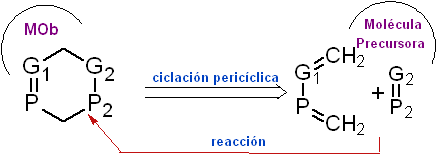
concerted pericyclic reaction, this is very common if in
Interconversion of Functional Groups (IGF).
It is the process by which the group(s) functional group(s) of the molecule subjected to a retrosynthetic analysis, is converted into another functional group, located in the structure of the synthon or synthetic equivalent (precursor molecule) that through a normal reaction of substitution, elimination, addition, rearrangement, oxidation or reduction, it will be transformed into the functional group of the molecule that is desired to be obtained.
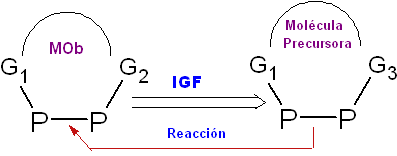
The reverse of an IGF is the chemical reaction. It is represented by the arrow: ![]() with the initials IGF or others that represent retrosynthesis operations, on it
with the initials IGF or others that represent retrosynthesis operations, on it
Addition of Functional Group (AGF ).
It is the addition of a new functional group in the structure of the precursor molecule (synthetic equivalent), which will become
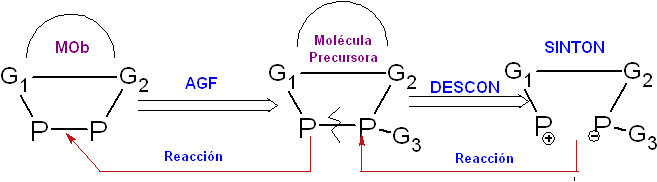
The purpose of introducing this new functional group is normally to make it easier for the structure of the precursor molecule (synthon, intermediate molecule, synthetic equivalent) to carry out a subsequent synthetic operation, which could be, for example: A simple disconnection, since the added functional group could stabilize the synthon structure resulting from the disconnection, activate or deactivate the molecule for some other chemical reaction thereof.
Withdrawal of Functional Group (RGF) or Suppression of Functional Group (SGF)
This retrosynthetic transformation operation allows the precursor molecule from which it will be prepared
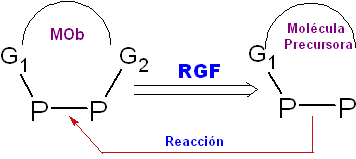
in the structure of
Synthon.
In general, this is the name given to the chemical species that almost always results from a disconnection of some bond of
Synthetic equivalent (parent molecule ).
In any case, a synthon is a chemical species that cannot be used directly in a chemical reaction, sometimes due to its instability and other times because it is a species that does not exist. For this, it will be necessary to previously carry out an abstraction process on the structure. synthon, to get to justify its existence through another molecule called synthetic equivalent. So that the latter, the synthetic equivalent, or precursor molecule, is a real molecule that acts like the synthons generated by the disconnection effected in
tautomerization.
It allows to explain the validity of a tautomer that is in equilibrium with a precursor molecule and whose structure allows to explain and understand a later synthetic operation in the synthesis design.