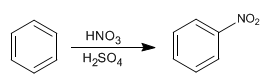
The benzene reacts with the nitric-sulfuric mixture adding nitro groups.
The electrophile in this reaction is the nitronium cation. NOT 2 + . The concentrations of this cation in nitric acid are too low to nitrate benzene, so it is necessary to add sulfuric acid.
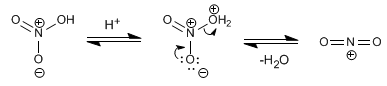
Mechanism for the nitration of benzene:
Stage 1. Attack of benzene on the nitronium cation 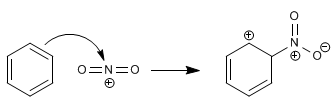
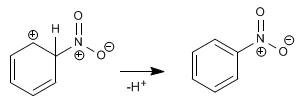
Stage 2. Recovery of aromaticity by loss of a proton