Name the following derivatives of benzene: 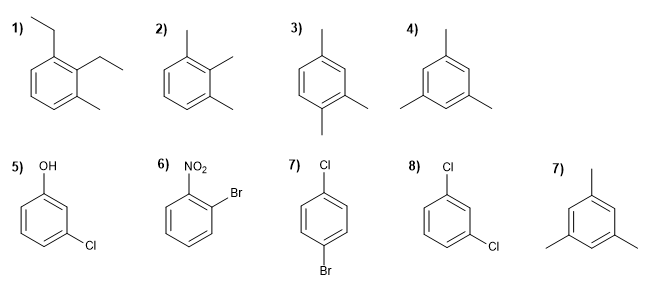
SOLUTION:
Molecule 1.
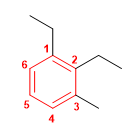
1. Main chain: benzene
2. Numbering: the substituents must take the minor locants, and in addition, the minor locants are assigned to the groups that come before them in alphabetical order (ethyl before methyl)
3. Substituents: 1,2-ethyl and 3-methyl.
4. Name: 1,2-Diethyl-3-methylbenzene
Molecule 2.
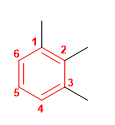
1. Main chain: benzene
2. Numbering: the substituents must take the minor locators.
3. Substituents: methyls in position 1,2,3 .
4. Name: 1,2,3-Trimethylbenzene
Molecule 3.
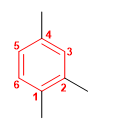
1. Main chain: benzene
2. Numbering: the substituents must take the minor locators.
3. Substituents: methyls in position 1,2,4 .
4. Name: 1,2,4-Trimethylbenzene
The ring is numbered so that the substituents take the lowest locants. In the event of a tie, the alphabetical order is taken into account.
Molecule 4.
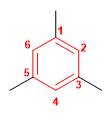
1. Main chain: benzene
2. Numbering: start with a methyl and number in any direction.
3. Substituents: methyls at 1,3,5 .
4. Name: 1,3,5-Trimethylbenzene
Molecule 5. 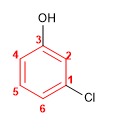
1. Main chain: benzene
2. Numbering: the numbering starts at the chlorine (it goes alphabetically first) and continues along the shortest path towards the hydroxyl.
3. Substituents: chlorine in position 1 and hydroxy in position 3 (meta position)
4. Name: 1-Chloro-3-hydroxybenzene ( m-Chlorohydroxybenzene)
Molecule 6.
1. Main chain: benzene
2. Numbering: Numbering starts on the bromine (alphabetical preference)
3. Substituents: bromo in position 1 and nitro in position 3 (ortho position)
4. Name: 1-Bromo-3-nitrobenzene ( o-Bromonitrobenzene)
Molecule 7. 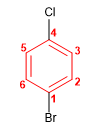
1. Main chain: benzene
2. Numbering: Starts at bromine (alphabetical preference over chlorine)
3. Substituents: bromo in 1 and chlorine in 4 (position para)
4. Name: 1-Bromo-4-chlorobenzene (p -Bromochlorobenzene)
Molecule 8. 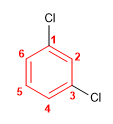
1. Main chain: benzene
2. Numbering: lowest possible locants to chlorines.
3. Substituents: chlorine in position 1,3 .
4. Name: 1,3-Dichlorobenzene (m-Dichlorobenzene)