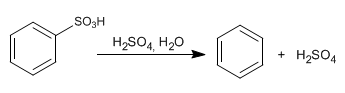The reaction of benzene with a solution of sulfur trioxide in sulfuric acid produces benzenesulfonic acids. 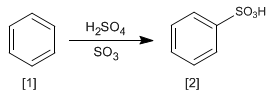
The mechanism of sulfonation takes place with the following stages:
Stage 1. Attack of benzene on sulfur trioxide 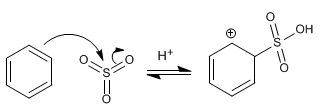
Stage 2. Recovery of aromaticity by loss of a proton. 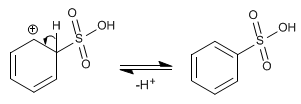
The sulfonation mechanism is reversible, which makes it possible to eliminate the -SO3H group by treatment with aqueous sulfuric acid. This property is used to protect benzene positions, occupying them with the -SO3H group.