Acyloinic condensation transforms esters into alpha-hydroxyketones. This reaction is carried out with sodium metal in an inert solvent. 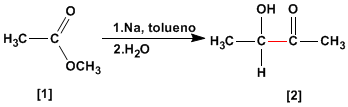
Mechanism
In the mechanism of the reaction, the sodium metal reacts with the ester to form an anion-radical, which dimerizes to form the dianion. 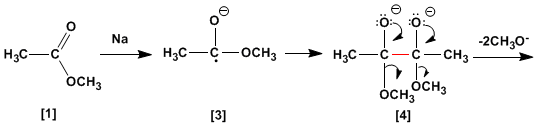
The diketone is formed by loss of two equivalents of methoxide. Reaction with two equivalents of sodium metal converts the diketone to the enodiolate, which generates an enodiol on aqueous workup. 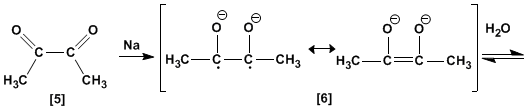
The last stage is the interconversion between enol and ketone, known as keto-enol tautomerism. 
Acyloin condensation is also an important method for preparing medium and large cycles. 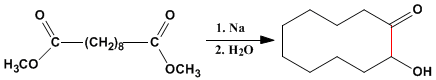
References
1. N. Allinger, Org. Synth. IV:840(1963)
2. J.-P. Sauvage, Acc. Chem. Res. 1990, 23, 319-327.
3. SM McElvain, Org. react. 1948, 4, 256-268.
4. M. Makosza and k. Grela, Synlett. 1997:267.