The ketone reaction with peracids produces esters . The oxygen of the peracid is inserted between the carbonyl carbon and the alpha carbon of the ketone. This reaction was described by Adolf von Baeyer and Victor Villiger in 1899. 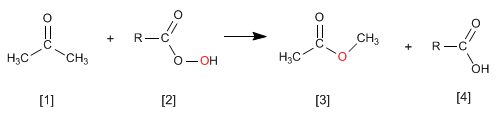
Cyclic esters (lactones) are obtained from cyclic ketones. 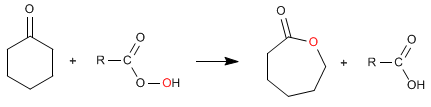
The Baeyer Villiger mechanism begins with the nucleophilic attack of the peracid on the carbonyl, followed by migration of the substituent from the carbonyl group to the oxygen of the peracid.
Step 1. Addition of the peracid to the carbonyl 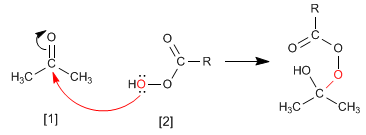
Stage 2. Migration of the substituent from carbonyl carbon to oxygen (red) 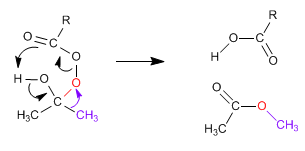
When the ketone has two different substituents, the more substituted one migrates better. There is a migration order that helps us to decide which substituent will join the oxygen of the peracid.
Migration order: H > tertiary carbon > cyclohexyl > secondary carbon » phenyl > primary carbon > methyl 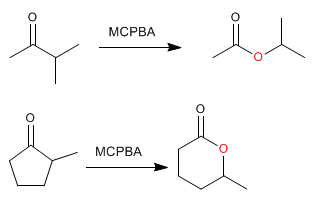
As can be seen in the order of migration, the group that best migrates, due to its small size, is hydrogen, therefore, when treating aldehydes with peracids, hydrogen migration occurs, forming carboxylic acids. 
MCPBA (Meta-Chloroperoxybenzoic Acid) is a peracid widely used in the epoxidation of alkenes and also in Baeyer-Villger. The MCPBA formula is shown below. 