Diels-Alder is a stereospecific reaction, it produces a single diastereoisomer. To draw the stereoisomer formed in a Diels-Alder, three rules are followed:
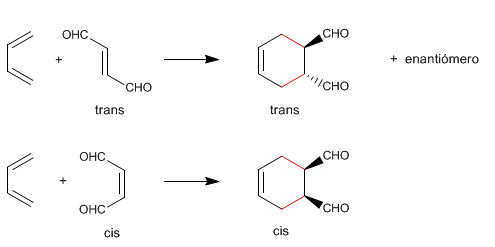
1. Diels-Alder preserves the stereochemistry of the dienophile .
If the alkene is cis, the substituents remain cis in the final product.
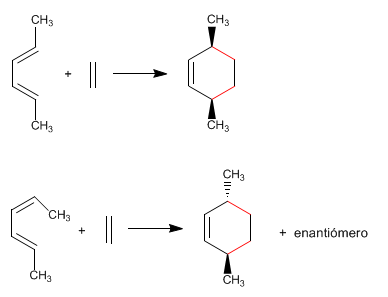
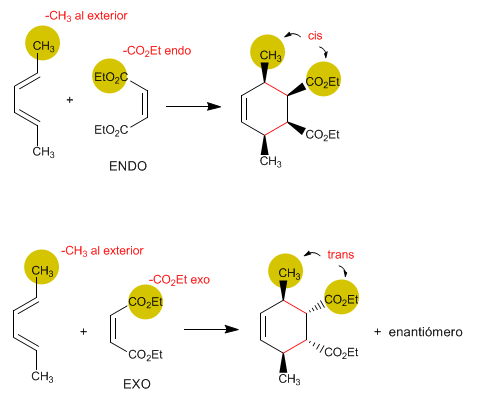
2. The Diels-Alder reaction preserves the stereochemistry of the diene.
If the substituents are located on the outside of the diene, they remain cis in the final adduct. If one substituent is on the inside and the other on the outside, they will remain trans in the product.
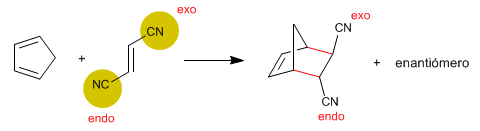
3. Diels-Alder is an ENDO reaction.
Alkene substituents that approach endo are cis with respect to diene substituents that are oriented outward.
The endo product is kinetic and is obtained at moderate temperature. Due to its greater stability, the exo product is thermodynamic and is obtained at a higher temperature.
In the case of using cyclic dienes, bicycles are obtained. In the endo approach, the alkene substituents are endo placed on the bicycle (on the opposite side of the bridge). The groups that approach exo, stay exo on the bike (oriented towards the bridge).