Draw chiral molecules that meet the following characteristics. Mark each center of chirality with an asterisk.
a) Chloroalkane of formula C5H11Cl
b) Alcohol of the formula C6H14O
SOLUTION:
a) The formula C5H11Cl has no unsaturations. The isomers will be straight or branched chain alkanes.
For the compound to be chiral it is necessary that it contain a carbon attached to four different substituents.
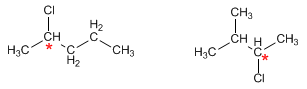
* Carbon attached to four different substituents (chiral center)
b) Formula C6H14O is saturated (complies with the formula CnH2n+2). It is an alcohol with a carbon chain without double bonds or cycles.
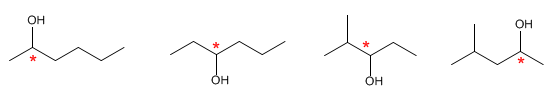
There are four chiral isomers that meet the previous formula, but there are more, which you can post in the stereochemistry forum.