Name the following compounds by assigning the absolute configuration (R) or (S) 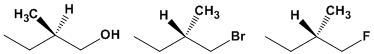
Solution 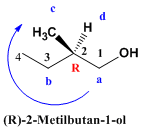 We choose the longest main chain (4 carbons). It is numbered starting with the carbon of the hydroxyl group (-OH) which is the functional group of the molecule. In position 2 there is a chiral center whose notation must be included in the name of the compound. We give priorities to the groups that start from the asymmetric carbon by atomic numbers, the clockwise rotation, with the group with the least priority at the bottom, indicates the R notation of the chiral center. The name of the molecule is composed of the notation of the chiral center, in parentheses, followed by the name of the compound.
We choose the longest main chain (4 carbons). It is numbered starting with the carbon of the hydroxyl group (-OH) which is the functional group of the molecule. In position 2 there is a chiral center whose notation must be included in the name of the compound. We give priorities to the groups that start from the asymmetric carbon by atomic numbers, the clockwise rotation, with the group with the least priority at the bottom, indicates the R notation of the chiral center. The name of the molecule is composed of the notation of the chiral center, in parentheses, followed by the name of the compound. 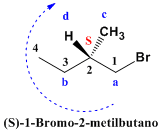 The compound is numbered so that the substituents (bromo and methyl) take the lowest locants. The name of the compound is 1-Bromo-2-methylbutane. However, it is necessary to indicate the notation of the chiral center that the molecule has in its position 2. We give priorities to the groups that start from the asymmetric carbon, “a” for the chain that reaches the bromine, “b” for the ethyl, “ c” for methyl and the lowest priority group is hydrogen to which we assign the letter “d” for having the lowest atomic number. The group "d" comes out towards us (wedge) and the notation of the center is contrary to the turn. Thus, the clockwise rotation implies an S notation.
The compound is numbered so that the substituents (bromo and methyl) take the lowest locants. The name of the compound is 1-Bromo-2-methylbutane. However, it is necessary to indicate the notation of the chiral center that the molecule has in its position 2. We give priorities to the groups that start from the asymmetric carbon, “a” for the chain that reaches the bromine, “b” for the ethyl, “ c” for methyl and the lowest priority group is hydrogen to which we assign the letter “d” for having the lowest atomic number. The group "d" comes out towards us (wedge) and the notation of the center is contrary to the turn. Thus, the clockwise rotation implies an S notation. 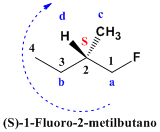 We assign priorities to the chiral center (carbon 2). The chain that reaches fluorine has priority “a”, since the atomic number of fluorine is higher than that of carbon. Ethyl beats methyl for having a longer chain and takes priority “b”. Hydrogen is the group with the lowest priority, since its atomic number is 1. The position of group “d” on the wedge tells us that the notation is anti-spin. Clockwise rotation, but notation S.
We assign priorities to the chiral center (carbon 2). The chain that reaches fluorine has priority “a”, since the atomic number of fluorine is higher than that of carbon. Ethyl beats methyl for having a longer chain and takes priority “b”. Hydrogen is the group with the lowest priority, since its atomic number is 1. The position of group “d” on the wedge tells us that the notation is anti-spin. Clockwise rotation, but notation S.