IUPAC names alkanoyl halides by replacing the -oic acid ending with an equal number of carbons per -oil . In addition, the word acid is replaced by the corresponding halogen, named as salt.
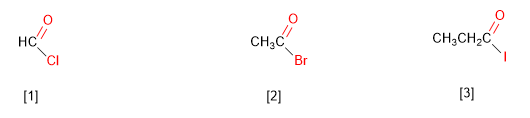
[1] Methanoyl chloride
[2] Ethanoyl bromide
[3] Propanoyl iodide
The longest chain containing the functional group is taken as the main chain. Numbering is done by giving the lowest locant to the carbon of the halide.

[4] 3-Methylhex-3-enoyl chloride
[5] 4-Chloro-3-ethylpentanoyl bromide
This functional group has priority over amines, alcohols, aldehydes, ketones, nitriles and amides (which must be named as substituents). Only carboxylic acids, anhydrides and esters have priority over it.

[6] 4-Hydroxy-3-methylpentanoyl chloride
[7] Bromide. 2-bromo-5-oxoheptanoyl
When in the molecule there is a priority group to the halide (carboxylic acid, anhydride, ester), the halide is named as: halocarbonyl.......

[8] 5-Chlorocarbonylhexanoic acid
[9] 4-Bromocarbonylbutanoic acid
When the halide is attached to a ring, the cycle is taken as the main chain and ends in
-carbonyl .

[10] Cyclohexanecarbonyl chloride
[11] 3-Methylcyclopentanecarbonyl bromide