Name the following alkynes according to IUPAC rules. 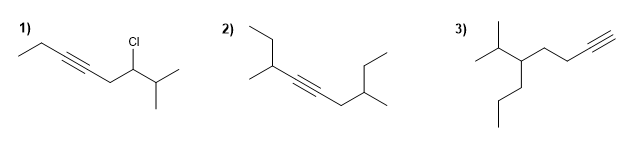
SOLUTION:
Molecule 1.
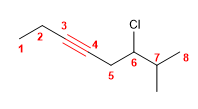
1. Main chain: 8-carbon chain (octino)
2. Numbering: start from the left end so that the triple bond takes the lowest locant
3. Substituents: chlorine in position 6 and methyl in 7 .
4. Name: 6-Chloro-7-methyloct-3-yne
Molecule 2. 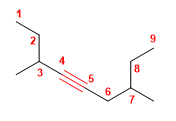
1. Main chain: the longest chain containing the triple bond has 9 carbons (nonine)
2. Numbering: start from the left end to give the locating triple bond 4 . If we number from the right the locator is 5 .
3. Substituents: methyls in position 3,7
4. Name: 3,7-Dimethylnon-4-yne
Molecule 3.
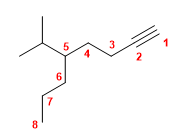
1. Main chain: 8-carbon chain (octino)
2. Numbering: lowest locant to the triple bond.
3. Substituents: isopropyl in position 5
4. Name: 5-Isopropyloct-1-yne
The triple bond has priority over the substituents, therefore, it must be contained in the main chain and must be numbered from the end of the chain that gives it the lowest locant.