Draw the structural formula and give the name of all the alkynes with the formula C5H8
SOLUTION:
Compounds with the formula C5H8 have 2 unsaturations, since they lack 4 hydrogens to fulfill the formula of alkanes, CnH2n+2 . In this problem the unsaturations are due to the presence of a carbon-carbon (alkyne) triple bond.
First we will build linear chains, and we will change the triple bond position. Next, we will build molecules with branches. Cyclic compounds with a triple bond are not valid, since they would have 3 unsaturations. Furthermore, there are no cyclic alkynes with less than 8 members.
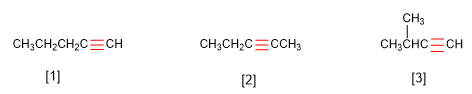
[1] Pent-1-yne
[2] Pent-2-yne
[3] 3-Methylbutyne