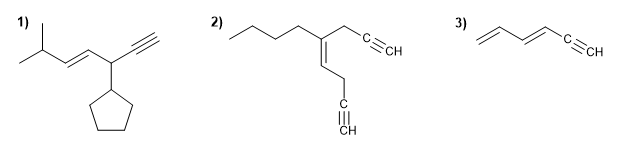
Name the following hydrocarbons with double and triple bonds.
SOLUTION:
Molecule 1.
1. Main chain: longest that contains the double and triple bond (hept-4-ene-1-yne).
2. Numbering: double and triple bond with the minor locants.
3. Substituents: 3-cyclopentyl and 6-methyl.
4. Name: 3-Cyclopentyl-6-methylhep-4-ene-1-yne
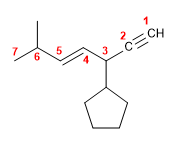
Molecule 2.
1. Main chain: the chain containing the double and triple bonds (oct-4-ene-1,7-diyno).
2. Numbering: since the multiple bonds are at the same distance from both ends ( 1,4,7 ), we number so that the butyl takes the smallest locant ( 4 ).
3. Substituents: 4- position butyl.
4. Name: 4-Butyloct-4-ene-1,7-diyno
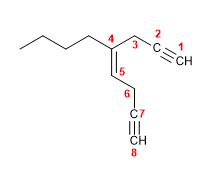
Molecule 3.
1. Main chain: 6 carbons with two double bonds and one triple. (hexa-1,3-diene-5-yne)
2. Numbering: the double bond has preference over the triple when numbering.
3. Substituents: none.
4. Name: Hexa-1,3-diene-5-yne