Rule 1. Alkynes respond to the formula $C_nH_{2n-2}$ and are named by replacing the suffix -ane of the alkane with the same number of carbons by -yne.
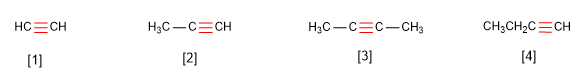
[1] Ethyne
[2] Propyne
[3] But-2-yne
[4] But-1-yne
Rule 2. The longest chain that contains the triple bond is chosen as the main chain. The numbering must give the lowest locants to the triple bond.
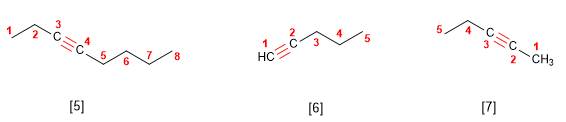
[5] Oct-3-yne
[6] Pent-1-yne
[7] Hex-2-yne
Rule 3. When the molecule has more than one triple bond, the chain that contains the greatest number of triple bonds is taken as the main one and is numbered from the end closest to one of the multiple bonds, ending the name in -diino , trill, etc.
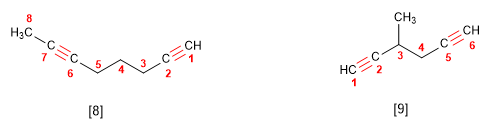
[8] Oct-1,6-diino
[9] 3-Methylhexa-1,5-diyne
Rule 4. If the hydrocarbon contains double and triple bonds, proceed as follows:
1. The main chain is taken as the one that contains the largest possible number of multiple bonds, regardless of whether they are double or triple.
2. It is numbered so that the links as a whole take the lowest locants. If there is a double bond and a triple at the same distance from the ends, the double takes precedence.
3. If the compound has a double bond and a triple, the name ends in -ene-yne; if you have two doubles and one triple, -diene-yne; with two triples and one double the ending is, -eno-diino
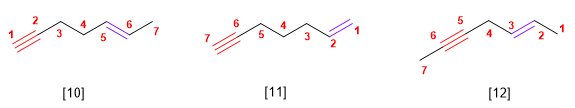
[10] Hept-5-ene-1-yne
[11] Hept-1-ene-6-yne
[12] Hept-2-ene-5-yne