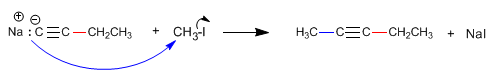
As we saw in the previous point, terminal alkynes have acidic hydrogens that can be removed by strong bases, forming acetylides (conjugate base of the alkyne). Acetylides are good nucleophiles and give mechanisms of nucleophilic substitution with primary substrates.
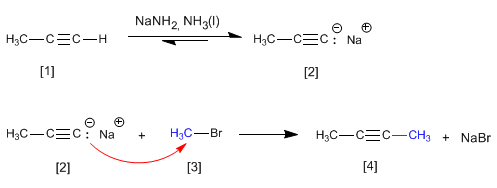
Propyne reacts with sodium amide in liquid ammonia to form propynyl sodium. In the second stage, sodium propynyl attacks methyl bromide as a nucleophile to form 2-Butyne. In this reaction, the alkyne is alkylated, forming a carbon-carbon bond that increases the size of the carbon chain.
The formation of carbon-carbon bonds is of great importance in organic synthesis, allowing the construction of large molecules from smaller ones.
The alkylation reaction can only be carried out with primary haloalkanes. Thus, isopropyl bromide (secondary substrate) generates propene by reaction with propynyl sodium, through the E2 mechanism.
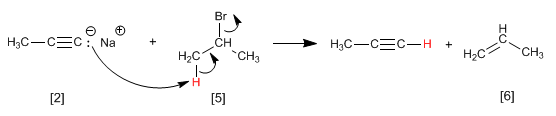
Example: Obtain 2-pentyne from acetylene
Step 1. Deprotonation of acetylene with sodium amide
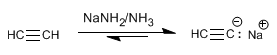
Step 2. Alkylation with ethyl iodide
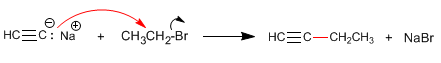
Step 3. Deprotonation of 1-Butyne
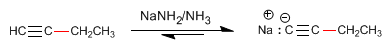
Step 4. Alkylation of 1-butynyl sodium with methyl iodide