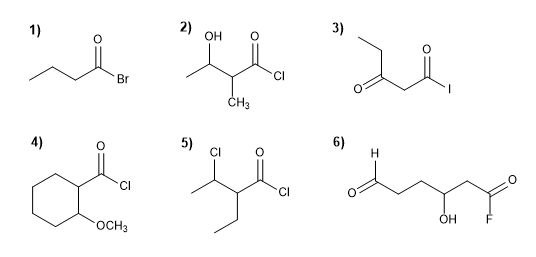
Write the IUPAC name of the following alkanoyl halides.
SOLUTION:
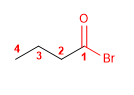
Molecule 1.
1. Main Function: Alkanoyl Halide
2. Numbering: Functional G. with lower locator
3. Substituents: no
4. Name : Butanoyl Bromide
2. Numbering: Functional G. with lower locator
3. Substituents: no
4. Name : Butanoyl Bromide
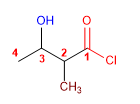
Molecule 2.
1. Main Function: Alkanoyl Halide
2. Numbering: Functional G. with lower locator
3. Substituents: 3-(hydroxy)alcohol, 2-methyl
4. Name: 3-hydroxy-2-methylbutanoyl chloride
2. Numbering: Functional G. with lower locator
3. Substituents: 3-(hydroxy)alcohol, 2-methyl
4. Name: 3-hydroxy-2-methylbutanoyl chloride
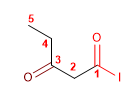
Molecule 3.
1. Main Function: Alkanoyl Halide
2. Numbering: Functional G. with lower locator
3. Substituents: ketone (oxo) in 3
4. Name: 3-oxopentanoyl iodide
3. Substituents: ketone (oxo) in 3
4. Name: 3-oxopentanoyl iodide
Molecule 4.
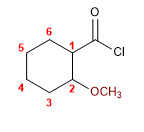
1. Main Function: Alkanoyl Halide
2. Numbering: functional G. with lower localizer (without numbering the halide carbon)
3. Substituents: 2-methoxy
4. Name: 2-Methoxycyclohexanecarbonyl chloride
2. Numbering: functional G. with lower localizer (without numbering the halide carbon)
3. Substituents: 2-methoxy
4. Name: 2-Methoxycyclohexanecarbonyl chloride
Molecule 5.
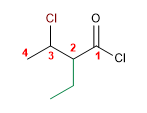
1. Main Function: Alkanoyl Halide
2. Numbering: Functional G. with lower locator
3. Substituents: 3-chloro and 2-ethyl
4. Name: 3-chloro-2-ethylbutanoyl chloride
2. Numbering: Functional G. with lower locator
3. Substituents: 3-chloro and 2-ethyl
4. Name: 3-chloro-2-ethylbutanoyl chloride
Molecule 6.
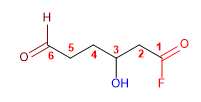
1. Main Function: Alkanoyl Halide
2. Numbering: Functional G. with lower locator
3. Substituents: alcohol (hydroxy) in 3 and aldehyde (oxo) in 6
4. Name: 3-hydroxy-6-oxohexanoyl fluoride