Hydroboration is the anti-Markovnikov hydration of an alkyne. A hindered borane (dicyclohexylborane or diisoamylborane) is used as a reagent, obtaining an enol that tautomerizes to aldehyde or ketone. Terminal alkynes give rise to aldehydes, internal ones to ketones.
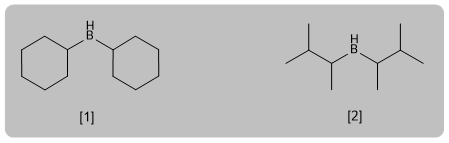
[1] Dicyclohexylborane (Cy2BH)
[2] Diisoamylborane
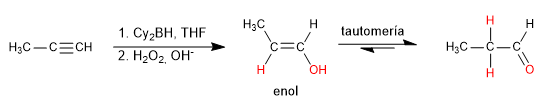
The mechanism of the reaction goes through the following steps:
Stage 1. Addition of the borane to the triple bond. Boron is attached to the least substituted carbon of the alkyne.
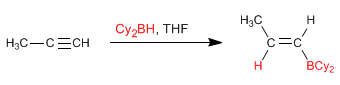
Stage 2. Oxidation of borane to alcohol
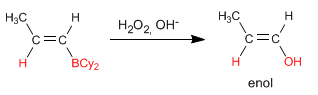
Stage 3. Keto-enol tautomerism
