The carbon-hydrogen bond of terminal alkynes is polarized and shows a slight tendency to ionize.
Propyne Hydrogen is weakly acid, with a pKa = 25. Using strong bases (NaH, LDA, NH 2 - ) it can be deprotonated, obtaining its conjugate base -propynyl sodium- a very basic and nucleophilic species.
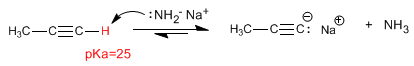
The propynyl sodium generated in the above reaction can act as a nucleophile attacking primary haloalkanes through the SN2 mechanism.
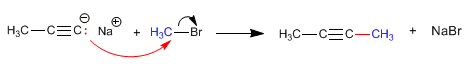
Bases derived from alcohols and water are too weak to deprotonate alkynes, with acid-base equilibria shifted far to the left.
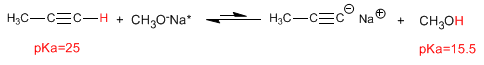
An acid-base equilibrium is shifted toward the conjugate acid with the higher pKa value.
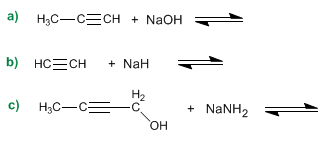
Example: complete the following acid-base equilibria