Aldehydes are named by replacing the -e ending of the corresponding alkane with -al . It is not necessary to specify the position of the aldehyde group, since it occupies the end of the chain (locant 1).
When the string contains two aldehyde functions, the suffix -dial is used.
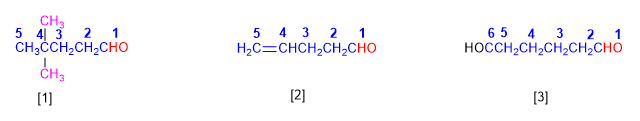
[1] 4,4-Dimethylpentanal
[2] Pent-4-enal
[3] Hexanodial
The -CHO group attached to a ring is called -carbaldehyde . The numbering of the cycle is carried out by giving locant 1 to the carbon of the cycle that contains the aldehyde group.

[4] Cyclohexanecarbaldehyde
[5] 3-Bromocyclopentanecarbaldehyde
Some common names for aldehydes accepted by IUPAC are:
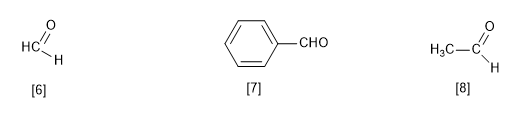
[6] Methanal (formaldehyde)
[7] Benzaldehyde
[8] Ethanal (acetaldehyde)
Ketones are named by replacing the -e ending of the alkane with the same chain length with -one . The longest chain that contains the carbonyl group is taken as the main chain and is numbered so that it takes the lowest locant.
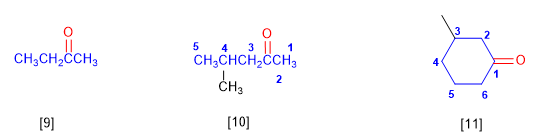
[9] Butanone
[10] 4-Methylpentan-2-one
[11] 3-Methylcyclohexanone
There is a second type of nomenclature for ketones, which consists of naming the chains as substituents, arranging them alphabetically, and ending the name with the word ketone .
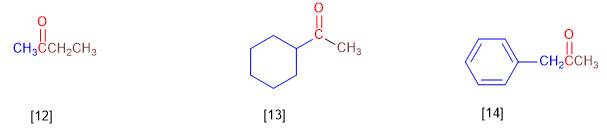
[12] Ethyl methyl ketone
[13] Cyclohexyl methyl ketone
[14] Benzyl methyl ketone
When the ketone acts as a substituent, it is named with the prefix -oxo. Priority groups to the ketone are carboxylic acids and their derivatives, as well as aldehydes.
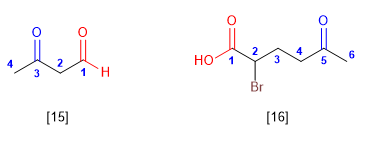
[15] 3-Oxobutanal
[16] 2-Bromo-5-oxohexanoic acid