 The a,b -unsaturated carbonyls are organic compounds that have a double bond between the a,b positions of an aldehyde or ketone.
The a,b -unsaturated carbonyls are organic compounds that have a double bond between the a,b positions of an aldehyde or ketone.
Propenal or acrolein is an a,b -unsaturated carbonyl. Its two conjugated double bonds give it special reactivity.
There are 4 important methods for the preparation of a,b -unsaturated: aldol condensation, halogenation of the a-carbon followed by elimination, oxidation of allylic alcohols, and Wittig.
Method 1. Prepare by aldol condensation the following compound. 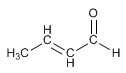
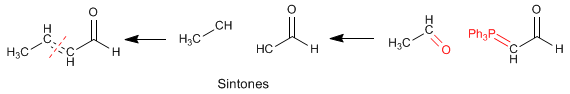
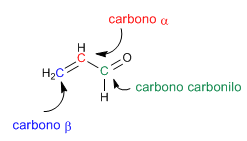
We employed retrosynthesis to prepare the compound. Being from the a,b -unsaturated family, it can be obtained by aldol condensation. 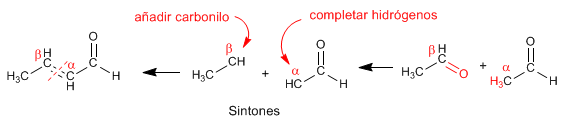 To obtain the reagents that form the a,b -unsaturated, it is broken by the double bond, obtaining the synthons (synthetic equivalents). The reagents are obtained by adding a carbonyl to carbon b and completing the missing hydrogens on carbon a .
To obtain the reagents that form the a,b -unsaturated, it is broken by the double bond, obtaining the synthons (synthetic equivalents). The reagents are obtained by adding a carbonyl to carbon b and completing the missing hydrogens on carbon a .
Example 2. Indicate how the following transformation can be carried out. 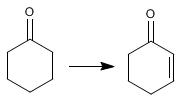
In a first step, the a position of the carbonyl is halogenated. In the second stage, an elimination is carried out that leaves us with the final product. 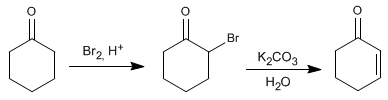
Method 3. Oxidation of allylic alcohols with manganese dioxide in acetone produces a,b -unsaturates 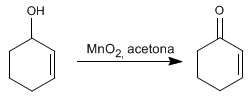
Method 4. Wittig reaction