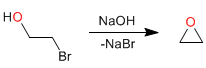
Mechanism:
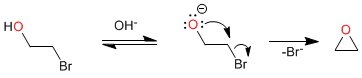
The first stage of the mechanism consists of an acid-base balance that depotifies the alcohol, generating a good nucleophile (alkoxide) that cyclizes in the second stage through an intramolecular S N 2 .
Alkene epoxidation: Peracids (MCPBA) react with alkenes to form oxiranes (epoxides).

Mechanism:
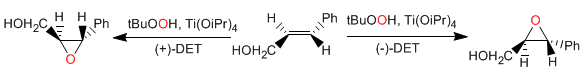
Sharples asymmetric epoxidation: Allyl alcohols react enantioselectively with titanium tetraisopropoxide, tert-butylhydroperoxide, and (+) or (-) diethyltartrate to give oxiranes.
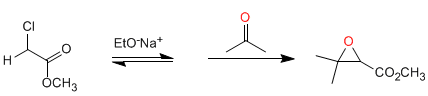
Darzens synthesis: The reaction starts from halogenated esters in alpha position, which form enolates in a basic medium. Reaction of the enolate ester with aldehydes or ketones, followed by intramolecular S N 2 , produces oxiranes.
Mechanism:
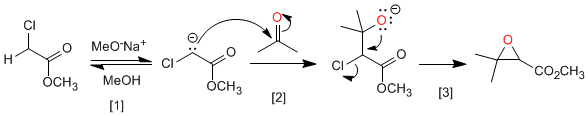
Ester deprotonation.
Nucleophilic attack of the enolate ester on the carbonyl.
Intramolecular nucleophilic substitution that generates oxirane.
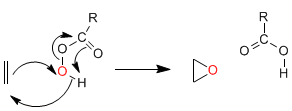
Corey synthesis (sulfur ylides): Sulfur ylides react with aldehydes or ketones to form oxiranes.
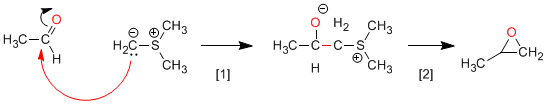
Nucleophilic attack of the ylide to the carbonyl.
Cyclization stage taking advantage of the capacity of sulfur as a leaving group.
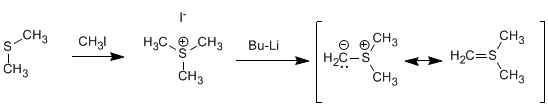
Sulfur ylide is prepared by methylation of dimethyl sulfide followed by treatment with strong base.