Carboxylic acids can be prepared using the following methods:
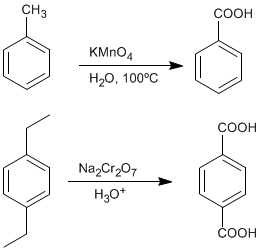
♦ Oxidation of alkylbenzenes: Carboxylic acids can be obtained from benzenes substituted with alkyl groups by oxidation with potassium permanganate or sodium dichromate.
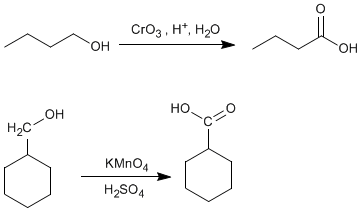
♦ Oxidation of primary alcohols: Carboxylic acids can be obtained by oxidation of primary alcohols. Jones oxidant, potassium permanganate, sodium dichromate can be used as reagents......
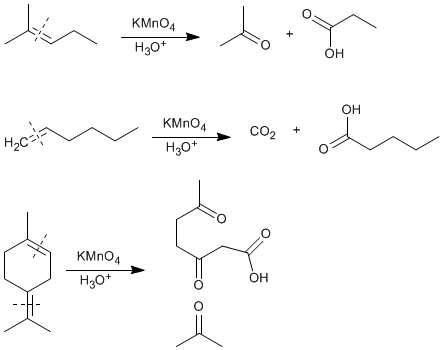
♦ Oxidation of alkenes : The oxidative cleavage of alkenes with oxidants such as potassium permangante or dichromate in acid media generates carboxylic acids when the alkene has a hydrogen on the sp 2 carbon. In the absence of hydrogen, ketones are formed, and terminal alkenes produce carbon dioxide.
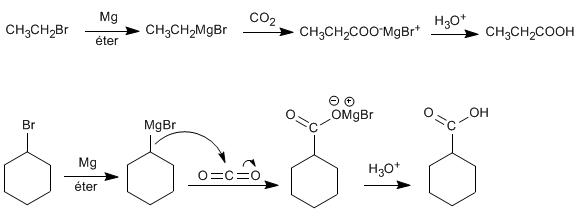
♦ Organometallics with CO2 : Grignard reagents (magnesium organometallics) react with carbon dioxide to form salts of carboxylic acids. A subsequent acid hydrolysis allows the conversion of these salts into the corresponding acid.
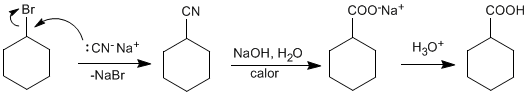
♦ Hydrolysis of nitriles: Primary and secondary haloalkanes react with sodium cyanide through S N 2 type mechanisms to form nitriles. Subsequent hydrolysis of the nitrile yields carboxylic acids. Haloalkanes with one carbon less than the acid to be obtained should be used.
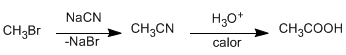
Nitrile hydrolysis can be carried out in a basic medium, generating a carboxylate that is protonated in a final acidification stage.