Aldehydes and ketones can be prepared by oxidation of alcohols, ozonolysis of alkenes, hydration of alkynes, and Friedel-Crafts acylation as major methods.
a) Ozonolysis of alkenes: Alkenes break down with ozone to form aldehydes and/or ketones. If the alkene has vinyl hydrogens it gives aldehydes. If it has two carbon chains it forms ketones. 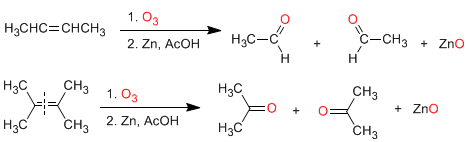
Ozonolysis of cyclic alkenes produces dicarbonyl compounds: 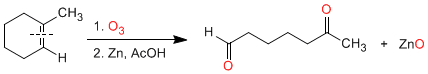
The terminal alkenes break to form methane, which is easily separated from the mixture due to its low boiling point. 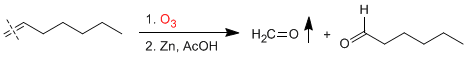
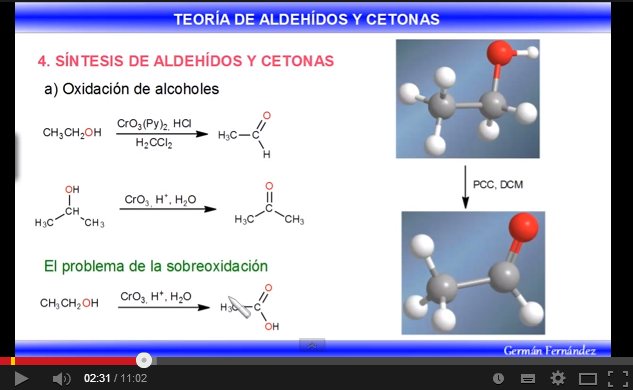
b) Oxidation of alcohols: Primary and secondary alcohols are oxidized to give aldehydes and ketones respectively. Precautions must be taken in the oxidation of primary alcohols, since they overoxidize carboxylic acids in the presence of oxidants containing water. In these cases, anhydrous reagents should be used, such as pyridine chlorochromate in dichloromethane (PCC), at room temperature. 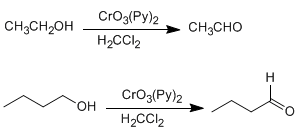
Secondary alcohols give ketones by oxidation. Permanganate, dichromate, chromium trioxide are used as oxidants. 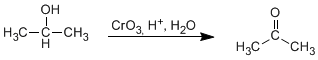
Oxidation involves the loss of two hydrogens from the alcohol. Tertiary alcohols cannot oxidize since they lack hydrogen on carbon. 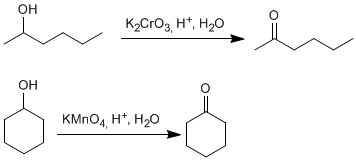
Allyl and benzyl alcohols are converted to aldehydes or ketones by oxidation with manganese dioxide in acetone. This reaction has a high selectivity and does not oxidize alcohols that are not in said positions. 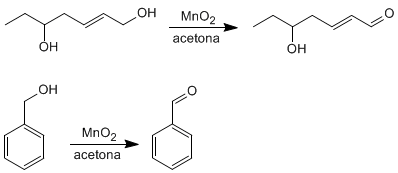
c) Alkyne hydration: Alkynes can be hydrated by Markovnikov, forming ketones, or antiMarkovnivov, to form aldehydes. 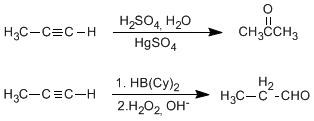
d) Friedel-Crafts acylation: The introduction of acyl groups into benzene allows the preparation of ketones with aromatic chains. 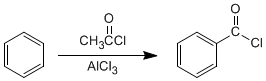
Video