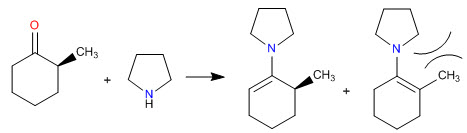
Enamine synthesis
As we saw in previous sections, the condensation of primary amines with aldehydes and ketones generates imines. In this section we will study the condensation of carbonyls with secondary amines that give enamines.
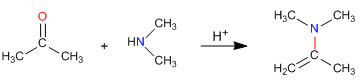
Enamine formation mechanism
After the initial attack of the secondary amine on the carbonyl, water is removed, forming the double bond between the carbonyl carbon and the alpha of the starting carbonyl.
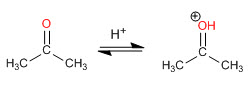
Step 1. Protonation of the carbonyl
Step 2. Nucleophilic attack of the secondary amine
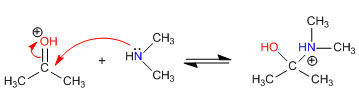
Stage 3. Acid-base balance
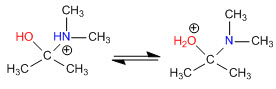
Stage 4. Loss of water
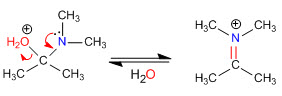
Stage 5. Elimination
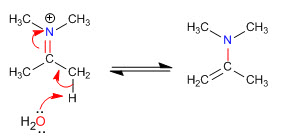
Steric hindrances make the least substituted enamines the most stable