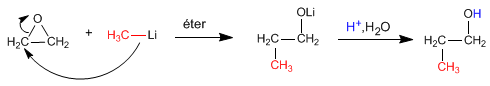
Alcohols can be obtained by opening epoxides (oxacyclopropanes). This opening can be done using organometallic reagents or the lithium aluminum reductant.
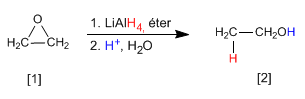
Oxacyclopropane [1] is transformed by reduction with lithium aluminum hydride in ethanol [2].
The mechanism The mechanism of the reaction begins with the attack of the hydride from the reducing agent on the positively polarized carbon of the epoxide, to end with the protonation of the alkoxide
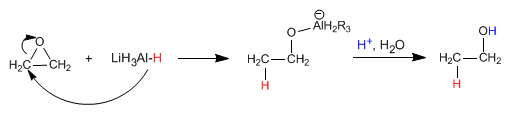
Grignard reagents (magnesium organometallics) and organolytics react with oxacyclopropane to give a primary alcohol.
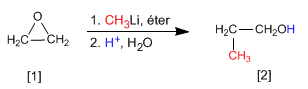
Methyllithium attacks oxacyclopropane [1] to form propan-1-ol [2]