Both sodium borohydride $(NaBH_4)$ and lithium aluminum hydride $(LiAlH_4)$ reduce aldehydes and ketones to alcohols.
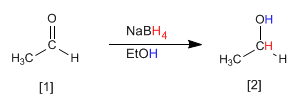
Ethanal is transformed by reduction with sodium borohydride to ethanol.
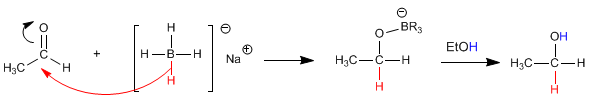
The mechanism occurs by attack of the hydride from the reductant on the carbonyl carbon. In a second stage the solvent protonates the oxygen of the alkoxide.
Lithium aluminum hydride works in an ether medium and transforms aldehydes and ketones into alcohols after an acid hydrolysis step.
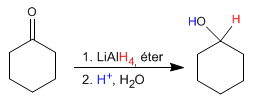
The mechanism is analogous to that of sodium borohydride.
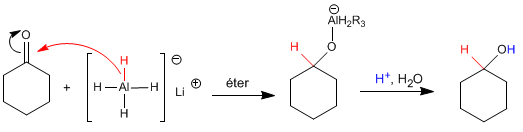
The lithium and aluminum reducer is more reactive than the boron one, it reacts with water and alcohols, releasing hydrogen. Therefore, it must be dissolved in aprotic media (ether).
The less reactive boron reducer decomposes slowly in protic media, allowing it to be used dissolved in ethanol or water.