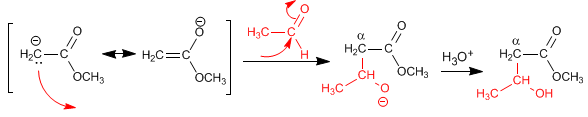
The esters have acidic hydrogens with pKa=25 in their position, which can be subtracted using bases. The conjugate base is an enolate ester, a highly nucleophilic species that attacks a varied number of electrophiles.
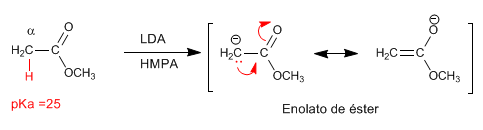
Reaction of ester enolates with primary haloalkanes
The ester enolate acts as a nucleophile, reacting with primary haloalkanes via the S N 2 mechanism. Secondary and tertiary haloalkanes give mostly eliminations, due to the significant basicity of the enolate.
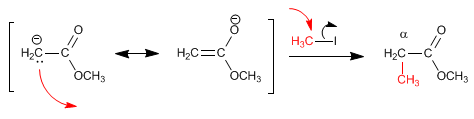
Reaction of ester enolates with oxacyclopropanes (epoxides)
The ester enolates attack and open oxacyclopropane. Oxacyclopropane contributes to the a-carbon of the enolate a chain with two carbons, which contains an -OH on the second carbon.
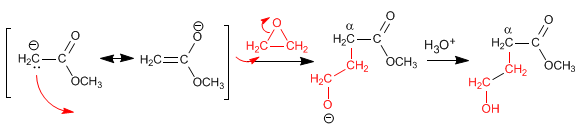
Reaction of ester enolates with aldehydes and ketones
The final product under suitable conditions can cyclize to form a lactone.