Alcohols can be obtained from haloalkanes by SN2 and SN1 reactions
Synthesis of alcohols using SN2
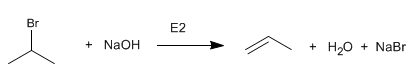
Primary haloalkanes react with sodium hydroxide to form alcohols. Secondary and tertiary haloalkanes eliminate to form alkenes.
Isopropyl bromide (secondary substrate) is removed by reacting with the hydroxide ion.
Synthesis of alcohols by SN1
Secondary and tertiary substrates react with water via the SN1 mechanism to form alcohols.
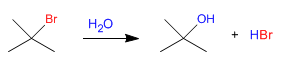
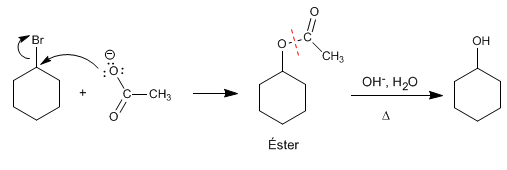
Ester hydrolysis
It is an interesting method to prepare alcohols from secondary haloalkanes. The haloalkane is converted into an ester by reaction with sodium acetate, to later be hydrolyzed in an acidic or basic medium, obtaining the alcohol.