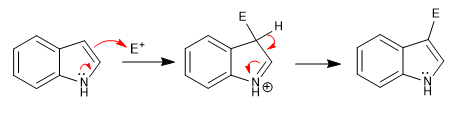
The attack on the electrophiles occurs from position 3, because the pyrrole ring is richer in charge density than the carbocycle. Said attack is favored by the transfer of the lone pair of nitrogen.
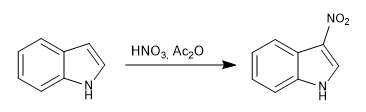
a) Nitration:
The indole is nitrated with the nitric mixture in acetic anhydride.
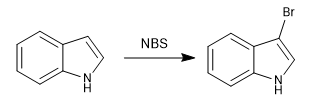
b) Halogenation
The halogenation of the indole is carried out with dilute halogens and cooling. NBS and NCS can also be used
c) Sulfonation: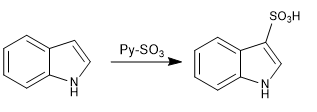
The indole sulfonation is performed with the SO3-pyridine complex under mild conditions to avoid possible polymerization side reactions.

d) Vilsmeier formulation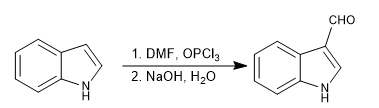
It allows to introduce formyl groups in position 3 of the ring. The reaction is carried out with dimethylfomamide and phosphorous oxytrichloride, followed by basic hydrolysis.

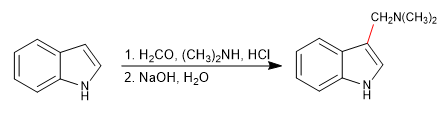
e) Mannich reaction
The Mannich reaction uses methanal and a primary or secondary amine in a hydrochloric medium as reagents.
