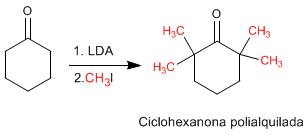
Enolates act as nucleophiles through carbon, attacking a large number of electrophiles (haloalkanes, epoxides, carbonyls, esters...). At this point we will focus on the reaction between enlolates and haloalkanes, which allows the addition of carbon chains to the a position of the chain.
Cyclohexanone is converted to 2-Methylcyclohexanone by treatment with LDA followed by methyl iodide. 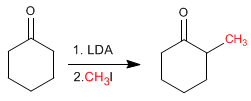
Stages of the mechanism by which cyclohexanone is alkylated:
Stage 1. Formation of the enolate 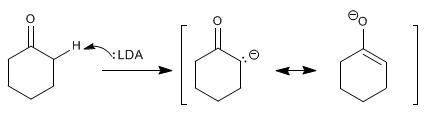
Stage 2. Nucleophilic attack of the enolate on the haloalkane (SN2 type reaction) 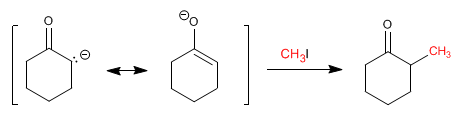
Alkylation reactions have two major problems.
1. Competition with aldol condensation. Carbonyls in a basic medium tend to condense to form aldols.
2. The reaction is difficult to control and tends to polyalkylate the carbonyl.