Oxiranes act as Lewis bases, protonating on oxygen in acidic media. The second type of reactivity is related to ring strain that favors opening reactions.
Isomerization to carbonyl compounds: Oxiranes in the presence of Lewis acids isomerize to form aldehydes or ketones. Thus, oxirane in the presence of BF 3 is transformed into acetaldehyde
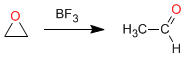
Example: Propose a mechanism for the following transformation:
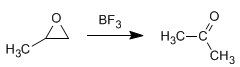
Solution - The BF 3 protonates the oxygen of the oxirane, turning it into a good leaving group and favoring the opening of the cycle. A rearrangement to a hydroxycarbocation will give the expected product.
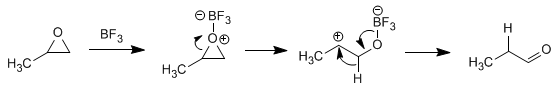
Opening of oxiranes in basic media - Good nucleophiles attack and open oxiranes on the least substituted carbon. The opening mechanism is of the S N 2 type and involves the attack of the nucleophile from the opposite side to oxygen, which behaves as a leaving group.
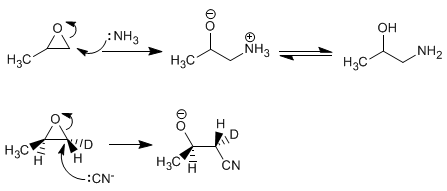
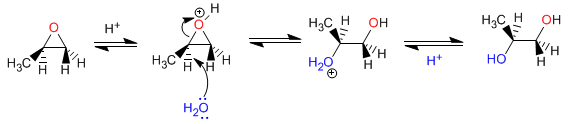
Opening of oxiranes in acidic medium - In acidic mediums, oxygen is protonated, favoring the nucleophilic attack on carbon. The opening occurs on the most substituted carbon. With H + /H 2 O anti diols are obtained.
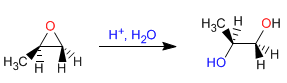
Mechanism:
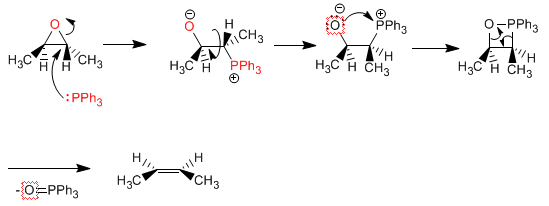
Deoxygenation of oxiranes: Oxiranes with trans substituents can be transformed into cis alkenes. Whereas, oxiranes with cis groups are converted to trans alkenes. Let's see an example: