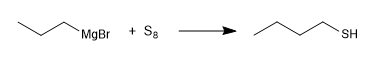Thiols are characterized by containing the -SH functional group. They are named by ending the main chain name in -thiol, analogous to alcohols whose ending is -ol
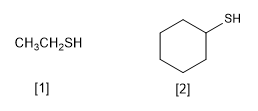
[1] Ethanethiol
[2] Cyclohexanethiol
Thiols have a higher acidity than alcohols due to the larger size of sulfur compared to oxygen. The pKa values are around 10-11, compared to alcohols that have values between 16-18.
However, the larger size of sulfur favors its polarizability and consequently its nucleophilicity. Thiols are much better nucleophiles than alcohols, their nucleophilicity being further enhanced by deprotonation.
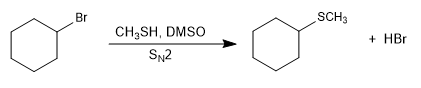
SN2 -type reactions become faster in the presence of a basic medium that generates the l-thiol (thiolate) salt.
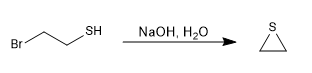
Mechanism:
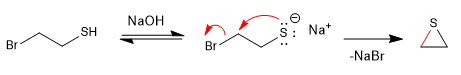
The thiols are prepared from sodium hydrogen sulfide and a primary or secondary haloalkane.
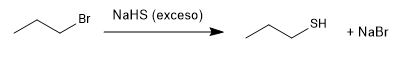
The excess hydrogen sulfide tries to minimize the side reaction whereby the propanethiol reattacks the propyl bromide to give a thioether.
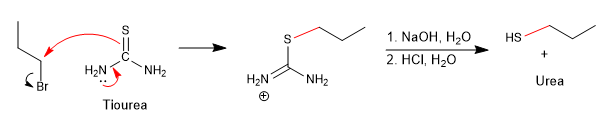
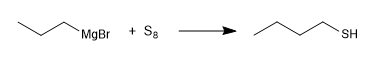
One way to avoid this side reaction is to use thiourea in combination with a haloalkane.

Another method of synthesis consists in the reaction of magnesium with elemental sulfur.