Name the following saturated hydrocarbons: 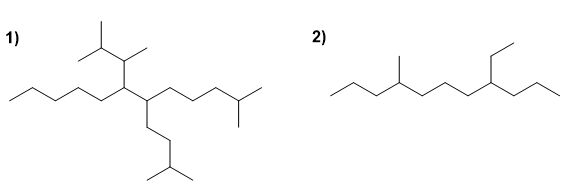
SOLUTION:
Molecule 1. 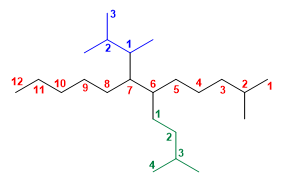
1. Main chain: the 12 carbon chain (undecane).
2. Numbering: methyl forces the main chain to be numbered starting from the right.
We number the side chains at positions 6 and 7 , giving locant 1 to the carbon that joins the main chain.
3. Substituents: 1,2-dimethylpropyl in position 7 and 3-methylbutyl in position 6 .
4. Name: 7-(1,2-dimethylpropyl)-2-methyl-6-(3-methylbutyl)dodecane ![]() Note that in complex substituents, quantity prefixes (di, tri, tetra...) are taken into account when alphabetizing. When the names become complex, it is convenient to use parentheses to separate some substituents from others.
Note that in complex substituents, quantity prefixes (di, tri, tetra...) are taken into account when alphabetizing. When the names become complex, it is convenient to use parentheses to separate some substituents from others.
Molecule 2.
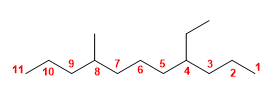
1. Main chain: chain of 11 carbons (undecane),
2. Numbering: whether we start the numbering from the right or from the left, we find a substituent in position 4 . In these cases, the substituent that comes first in the alphabetical order takes the lowest locant when numbering.
3. Substituents: 4-position ethyl and 8-position methyl.
4. Name: 4-Ethyl-8-methylundecane![]() When the substituents are at the same distance from both ends of the molecule, numbering begins with the end that assigns the lowest locant to the substituent that comes before it in alphabetical order.
When the substituents are at the same distance from both ends of the molecule, numbering begins with the end that assigns the lowest locant to the substituent that comes before it in alphabetical order.