For the following branched alkanes, choose the main chain, number it, and name the compound. 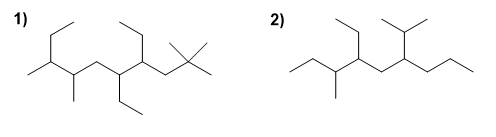
SOLUTION:
Molecule 1.
1. Main chain: the main chain is the longest
of the molecule (in red). If there are two chains of the same length, the one with the greatest number of substituents is taken as the main one.
Since the main chain has ten carbons, the name of the alkane ends in decane.
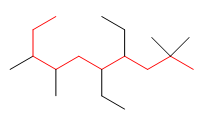
2. Numbering: we number the main chain starting from the end that has a carbon with substituents closest to it. Starting from the right we find substituents in 2, while starting the numbering from the left the first substituent is in 3 .
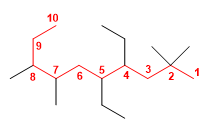
3. Construction of the name: We construct the name of the molecule, naming first the substituents preceded by the locants that indicate their position in the main chain, ending with the name of the main chain. The substituents are: methyls at 2,2,7,8; ethyls at 4.5 .
4,5-Diethyl-2,2,7,8-tetramethyldecane ![]() Quantity prefixes di, tri, tetra, penta are not considered when alphabetizing substituents. Thus, diethyl orders by the "e" of ethyl and tetramethyl orders by the "m" of methyl.
Quantity prefixes di, tri, tetra, penta are not considered when alphabetizing substituents. Thus, diethyl orders by the "e" of ethyl and tetramethyl orders by the "m" of methyl.
Molecule 2.
1. Main chain: The main chain has 9 carbons (nonane). Three substituents depart from it: ethyl, isopropyl and methyl. 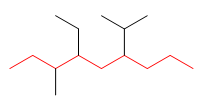
2. Numbering: the numbering of the main chain begins at the left end, since in position 3 we find the methyl. If we number from the right we will find the isopropyl in position 4 , which gives us larger locants. 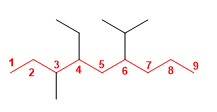
3. Construction of the name: We name the substituents in alphabetical order: ethyl, isopropyl and methyl. The name of each substituent is preceded by the locant indicating its position on the main chain: 4-ethyl, 6-isopropyl, 3-methyl. It ends with the name of the parent chain (nonane).
4-Ethyl-6-isopropyl-3-methylnonane![]() When building the name, the numbers of the letters are separated by hyphens. If there are several locators in a row, they are separated by commas. However, spaces are never left.
When building the name, the numbers of the letters are separated by hyphens. If there are several locators in a row, they are separated by commas. However, spaces are never left.