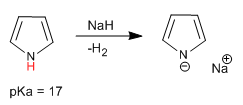
Pyrrole has an acid hydrogen on the nitrogen atom with pKa = 17. In the case of thiophene and furan, the acid hydrogens are located in position 2, although they have a much lower acidity than pyrrole.
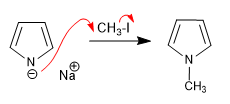
The pyrrolium anion, formed by deprotonating pyrrole, has an important ionic character that allows it to act as a nucleophile against a diverse group of carbon electrophiles.
Alkylation of the pyrrolium cation.
Acylation of the pyrrolium cation
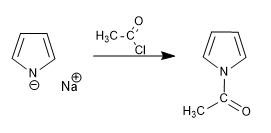
The ionic character of the pyrrolium ion is related to the base that generated it. Thus, sodium and potassium bases (sodium and potassium amides or hydrides) generate ionic pyrrolia. However, organometallics of lithium and magnesium or lithium amides produce pyrrolia with an important covalent character that keep the nitrogen couple blocked, producing the nucleophilic attack through the ring.
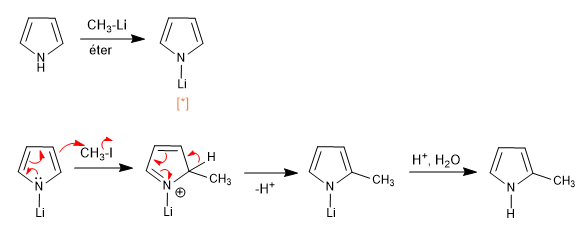
[*] Lithium or magnesium pyrrolides have an important covalent character, which blocks the nitrogen pair, producing the attack on the electrophile through the ring
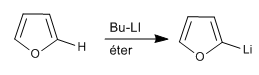
Thiophene and furan lack hydrogens on the heteroatom, but we can subtract hydrogens from the 2,5 positions by using strong bases (organometallics)
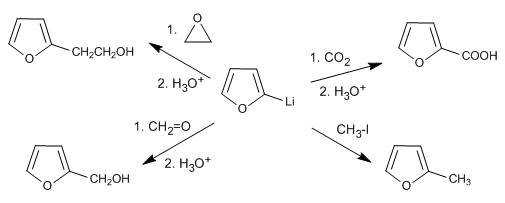
The reaction of the organolithic with different electrophiles allows chains to be added to position 2.
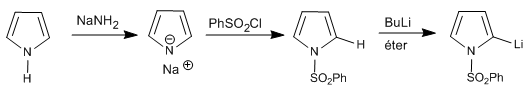
It is also possible to deprotonate the 2-position of a pyrrole, as long as the nitrogen does not have hydrogen.
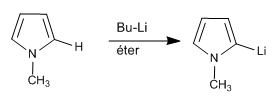
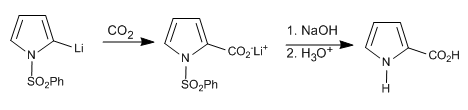
In the case of 1H-pyrroles, the following steps can be performed to deprotonate position 2.