The most characteristic property of carboxylic acids is the acidity of the hydrogen located on the hydroxyl group. The pKa of this hydrogen ranges from 4 to 5 depending on the length of the carbon chain.
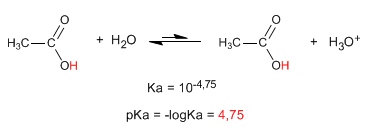
Carboxylic acids are relatively strong acids since they stabilize the charge of their conjugate base by resonance.
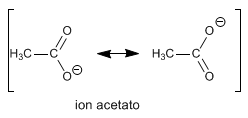
Electron-withdrawing substituents increase the acidity of carboxylic acids. High electronegativity groups remove charge due to the inductive effect of the carboxylic group, producing a decrease in the pKa of the acidic hydrogen.
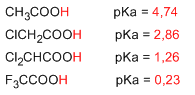
The inductive effect increases with the electronegativity of the halogen, with the proximity of the halogen to the carboxylic group, and with the number of halogens.
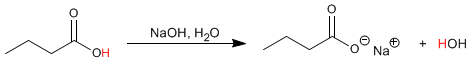
Carboxylic acids can be deprotonated with bases, such as NaOH, to form carboxylate salts. These salts are acceptable nucleophiles and can act in SN2 type mechanisms.