Amines have acidic hydrogens on the amino group. These hydrogens can be subtracted using strong bases (organometallic, metal hydrides) forming amides (amine bases).
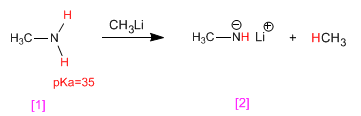
methylamine reacts with methyllithium, transforming into its conjugate base, lithium methylamide . For its part, methyllithium is transformed into its conjugate acid, methane.
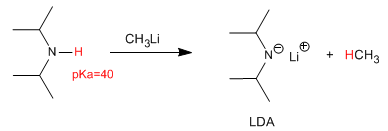
The deprotonation of diisopropylamine produces one of the most widely used bases in organic chemistry, lithium diisopropylamide (LDA).
However, the most important behavior of amines is basic. Amines are the most basic neutral organic substances.
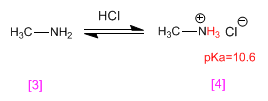
methylamine it is protonated becoming methylammonium chloride (ammonium salt) . Ammonium salts are the conjugate acids of amines and have pKas ranging from 9 to 11.
The basicity of amines depends on the inductive and steric effects. Thus, the pKa increases with the length of the carbon chain (inductive effect).
CH3NH2 pKa = 10.6
CH 3 CH 2 NH 2 pKa=10.8
(CH 3 ) 3 CNH 2 pKa=10.4
The carbon chain gives charge to the amino group, by inductive effect, increasing its basicity. The strong base has a weak conjugate acid, so the pKa goes up. But if the chain is very voluminous, steric effects begin to predominate, causing a decrease in pKa.