Magnesium organometallics at -78°C react with alkanoyl halides to form ketones. It is necessary to work at a low temperature to avoid adding a second equivalent of organometallic, in which case the product obtained would be an alcohol.

The mechanism occurs in two stages:
Stage 1. Addition of the organometallic to the halide
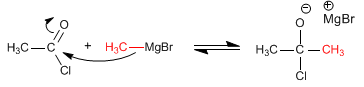
Stage 2. Elimination
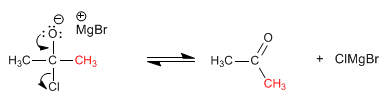
Cuprates also react with alkanoyl halides to form a ketone. Its lower reactivity allows it to work at room temperature.
